UFC 3-240-13FN
25 May 2005
always protect metals adequately, especially since cooling water is highly aerated
(oxygen saturated). Chemical corrosion inhibitors are used to provide protection from
corrosion of the metal components of cooling water systems. Table 4-10 shows criteria
for the selection of corrosion inhibitors. The principal strategy for a cooling system
corrosion protection program is to ensure protection of the metal in the heat exchanger
(metal that is the thinnest metal in the system). The secondary goal is to provide
protection from corrosion of the mild steel piping. When galvanized steel cooling towers
are part of the cooling system, specialized corrosion inhibitors are the best control
method. Galvanized steel is corroded at pH levels above 9.0 and below 6.0.
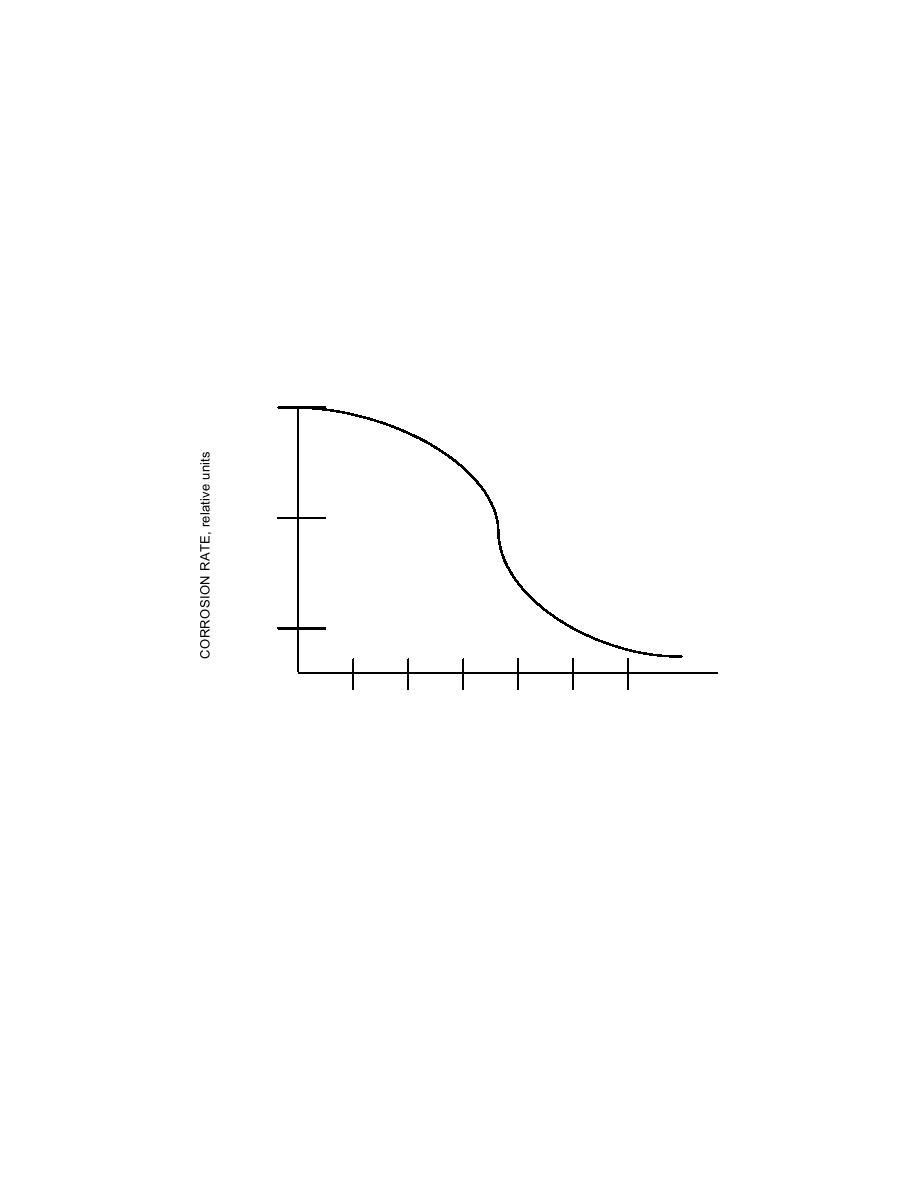
Figure 4-19. Effect of pH on Corrosion Rate of
Unprotected Mild Steel in Water
100
10
1
5
9
10
8
7
6
pH
129



 Previous Page
Previous Page
