UFC 3-240-13FN
25 May 2005
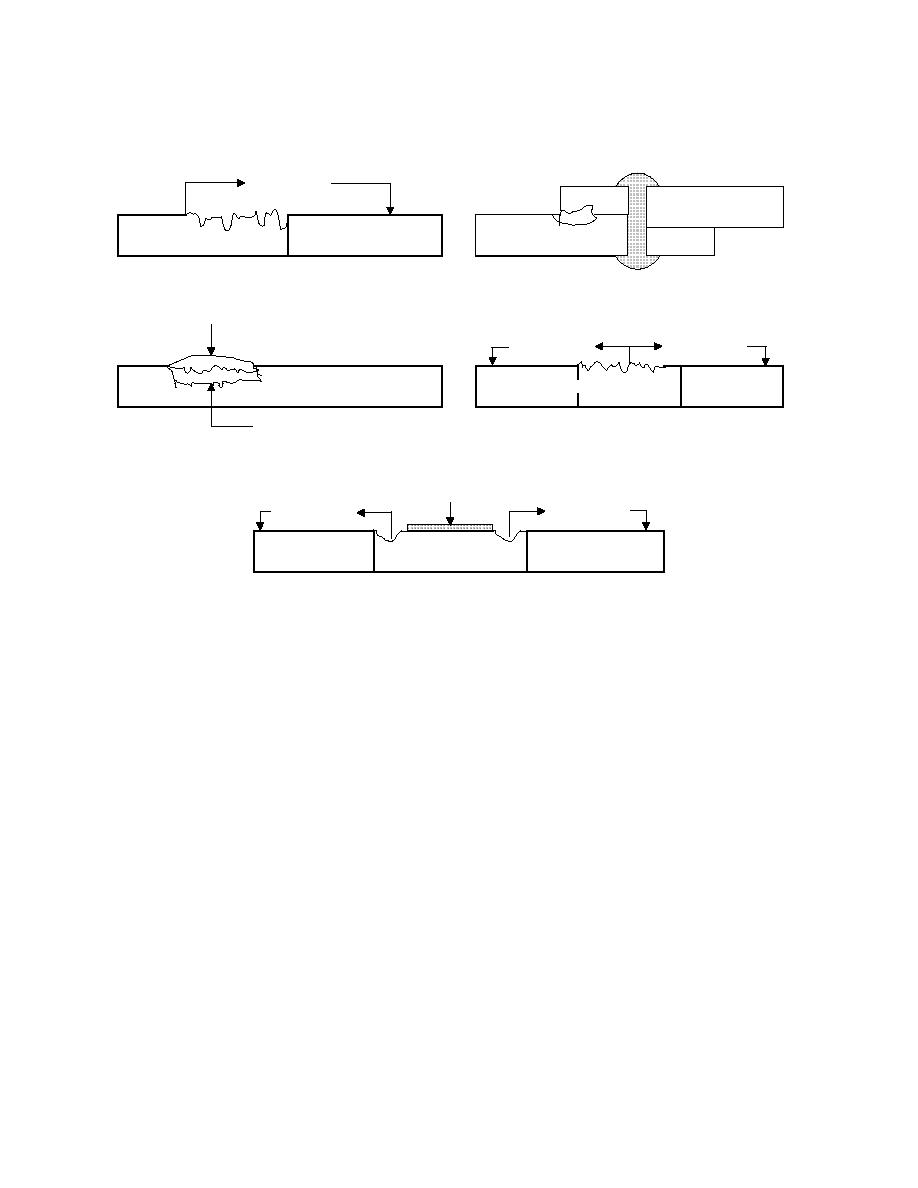
Figure 4-17. Forms of Corrosion
Current Flow
Steel
Steel
Copper
Steell
Stee
(A) Galvanic Corrosion
(B) Crevice Corrosion
Deposit
Current Flow
Current Flow
Cathode
Anode
Cathode
Corrosion
(C) Deposit Corrosion
(D) General Corrosion
Inhibitor
Film
Current Flow
Current Flow
Cathode
Cathode
Pit
Pit
Anode
(E) Pitting Attack
4-5.1
Galvanic Corrosion. See Figure 4-17 (A). This term refers to corrosion
that occurs when two different metals are coupled together. The metal with the least
resistance becomes the anode and will corrode due to the electrochemical reaction
produced. One of the most common instances of galvanic corrosion occurring in cooling
water systems results when mild steel and copper alloy metals are brought into contact
with one another (e.g., copper tubing attached to a mild steel tube sheet or brass valves
connected to mild steel or galvanized piping). As a result of the electrochemical
reaction, the copper is dissolved in the water and corrosion of copper alloy results. The
copper can also plate out (stick) on mild steel surfaces, setting up additional galvanic
cells. Another example is the electrochemical reaction that occurs when mild steel and
zinc (galvanizing) are coupled together at temperatures normally found in cooling tower
systems. The zinc becomes the anode and is corroded. Figure 4-18 shows the galvanic
series. Any coupling of a metal that is higher in the galvanic series with a metal or alloy
that is lower in the galvanic series results in electrochemical reaction in which the
"higher" metal functions as the anode or active metal.
125



 Previous Page
Previous Page
