TM 5-813-3/AFM 88-10, Vol 3
Table 2-3: Effectiveness of Various Unit Processes
breakpoint chlorination. To achieve the same disinfec-
for Reducing Chloroform Formation Potential (Cent `d)
tion ability of chlorine, 10 to 15 times the amount of
chloramines are needed or longer contact time is re-
quired. More chloramines are needed if high concen-
trations of organic material are found in the influent
water, Chloramines are easy to generate, feed, and
produce a persistant residual that will remain through
the water distribution system. Chloramines may be
produced by introducing ammonia to the water stream
prior to the addition of free chlorine. This process can
be optimized for minimum THM production and maxi-
mum disinfection. Recently however there has been
some concern over chloramine toxicity.
(4) Ultraviolet Radiation. Ultraviolet (UV) radi-
ation has undergone development, but has not been
used on a large scale for drinking water supply disin-
fection. Most of its uses include product or process
water disinfection where high purity, sterile water is
needed. UV radiation has been used to disinfect drink-
ing water at remotely located hotels and on cruise
ships. Few large scale water processing plants use UV
(1) Ozone. Ozone is an extremely powerful disin-
disinfection, although its application is feasible. UV
fectant that has been used in Europe either as a sole
disinfection does not leave a disinfectant residual and
disinfectant, or in conjunction with postchlorination
should be accompanied by postchlorination. Ultra-
to impart a persistent chlorine residual in the water
violet irradiation is also effective in oxidizing organic
distribution system. United States potable water
compounds in water, Water turbidity will inhibit the
plants have in the past used ozone to control taste and
effectiveness of UV disinfection.
odor. Today ozonation is being increasingly used as a
(5) UV and Ozone, Recently there has been some
primary disinfectant prior to rapid mixing, floccula-
experimentation in a combined UV and ozone con-
tion and filtration. Ozonation does not produce THMs.
.
tactor. Results from these tests show promise. How-
It is reduced to oxygen and does not leave any residual
ever, there is no known water treatment plant oper-
disinfectant. Hence, the need for postchlorination.
ating with this method of disinfection.
Ozone is generated electrically, as needed using the
electric discharge gap (corona) technique. Air or
2-8. Fluoride
adjustment.
oxygen stream, a cooling water stream and alternating
a. Health effects. An excessive fluoride concentra-
electric current are required. Efficient cooling is essen-
tion will damage the teeth of children using the water
tial to reduce thermal decomposition of ozone. Bubble
for extended periods. On the other hand, moderate
diffusers appear to be the most economic ozone con-
concentrations, 0.7- 1.2 mg/L, are beneficial to chil-
tractors available.
dren's teeth. Most natural waters contain less than the
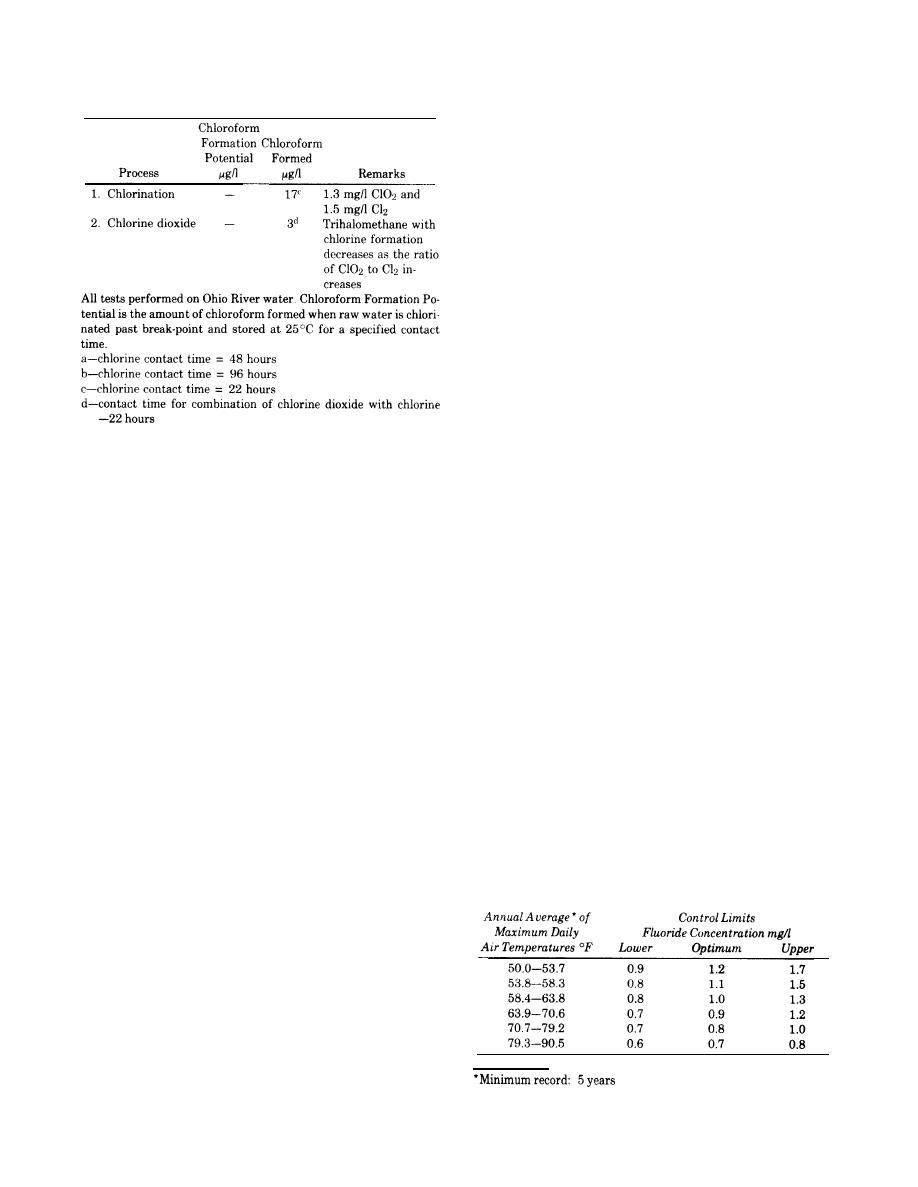
(2) Chlorine Dioxide, Chlorine dioxide is a highly
optimum concentration of fluoride. Upward adjust-
effective disinfectant producing minimal THMs in the
ment of the fluoride concentration can be achieved by
presence of their precursors. Chlorine dioxide uses in
application of a measured amount of a fluoride chem-
the United States have been limited to taste and odor
ical to the water. For installations where it is desirable
control although it has been used elsewhere as a pri-
and feasible to add fluoride, control limits and
mary disinfectant and is presently receiving more at-
optimum concentrations are as follows:
tention in the United States. The common method of
chlorine dioxide production is to react chlorine gas
from a conventional chlorinator with a sodium chlorite
solution. Following the mixing of the chlorine and so-
dium chlorite streams and prior to introduction into
the main stream the mixed stream is passed through a
packed column contactor to maximize chlorine dioxide
production. A major disadvantage of chlorine dioxide
is the formation of chlorate and chlorite which are po-
tentially toxic.
-.
(3) Chloramines, The use of chloramines as a dis-
infectant fell into disuse after the introduction of
2-17



 Previous Page
Previous Page
