UFC 3-240-13FN
25 May 2005
c) The carbon dioxide removal is: 31 - 2.1 = 28.9 ppm
2-2.6
heated to produce relatively pure water vapor that is then condensed to liquid water and
used for boiler feed. Evaporators are of several different types. The simplest is a tank of
water through which steam coils are passed to heat the water to the boiling point. To
increase efficiency, the vapor from the first tank may pass through coils in a second
tank of water to produce additional heating. Another type of evaporator operates under
a partial vacuum, lowering the boiling point of water and enabling evaporation at lower
temperatures. Following its production in the evaporator, water vapor is cooled and
becomes liquid water that is essentially pure water, without any dissolved solids. Using
evaporators may be economical where inexpensive steam is readily available as the
source of heat. Evaporators also have an advantage over deionization units when the
dissolved solids in the raw water are very high, such as on ocean-going vessels.
2-2.7
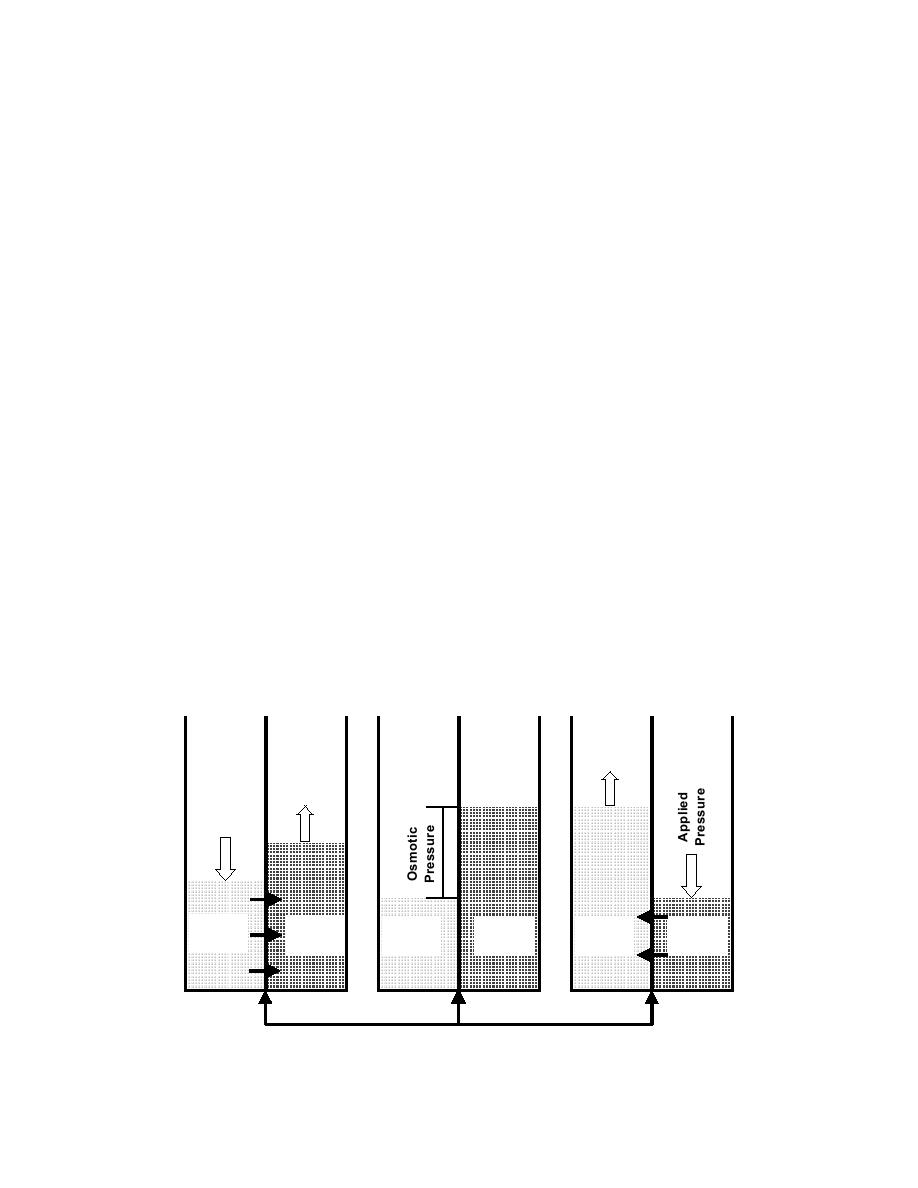
Reverse Osmosis (RO). This process is the opposite of osmosis. It
produces very pure water by separating dissolved minerals from the water. Water
pressure is used to push water though a membrane. The membrane allows only pure
water to pass through. Water thus produced is known as RO product water. All
dissolved solids, organics, and gases that do not pass through the membrane are
removed in the waste stream of RO reject water. Sufficient care must be taken to
protect the membrane from deposits, which reduce efficiency or plug the membrane.
This RO process is illustrated in Figure 2-7. An RO unit is shown in Figure 2-8.
Figure 2-7. RO Schematic
Osmotic
Osmosis
RO
Fresh
Salt
Fresh
Fresh
Salt
Salt
Water
Water
Water
Water
Water
Water
Membranes
29



 Previous Page
Previous Page
