UFC 3-240-13FN
25 May 2005
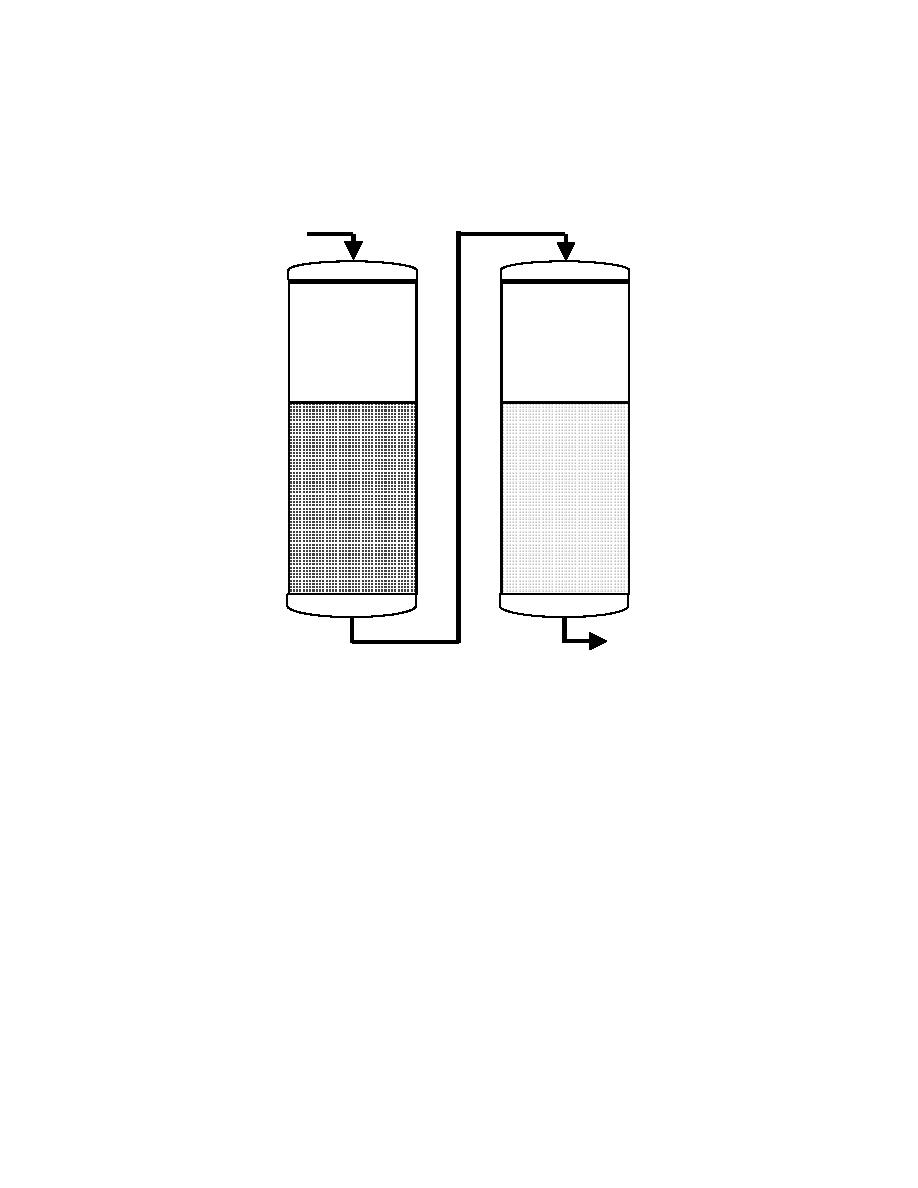
Figure 2-5. Anion Dealkalization Process
Raw
Water
Softener
Anion
Dealkalizer
Treated
Water
2-2.5.4.2
Split-Stream Dealkalization. In a split-stream dealkalization process, the
water is split into two parallel streams with one stream passing through a strong acid
cation exchanger (dealkalizer) and the other through a sodium zeolite softener.
Blending of the two product streams produces a soft water that is low in alkalinity. The
alkalinity reduction is described by the following equation, where "Z" is the zeolite resin
material:
→
Ca(HCO3)2
Z2Ca
+
2ZH
+
2H2CO3
Calcium
Calcium
Carbonic
Zeolite
Bicarbonate
Zeolite
Acid
The carbonic acid dissociates into carbon dioxide (CO2) and water (H2O). The
water is then passed into a decarbonator (degasifier or deaerator) to remove the CO2.
2-2.5.4.2.1 Free Mineral Acid Production. The strong acid cation exchange resin
replaces the sodium, calcium, and magnesium ions with hydrogen ions. Thus, due to
the presence of chlorides, sulfates, nitrates, and other anions, free mineral acids (FMA)
(i.e., hydrochloric, sulfuric) are produced. Adjustment of the pH level is necessary to
balance alkalinity with acidity (FMA) and form neutral water.
26



 Previous Page
Previous Page
