UFC 3-240-13FN
25 May 2005
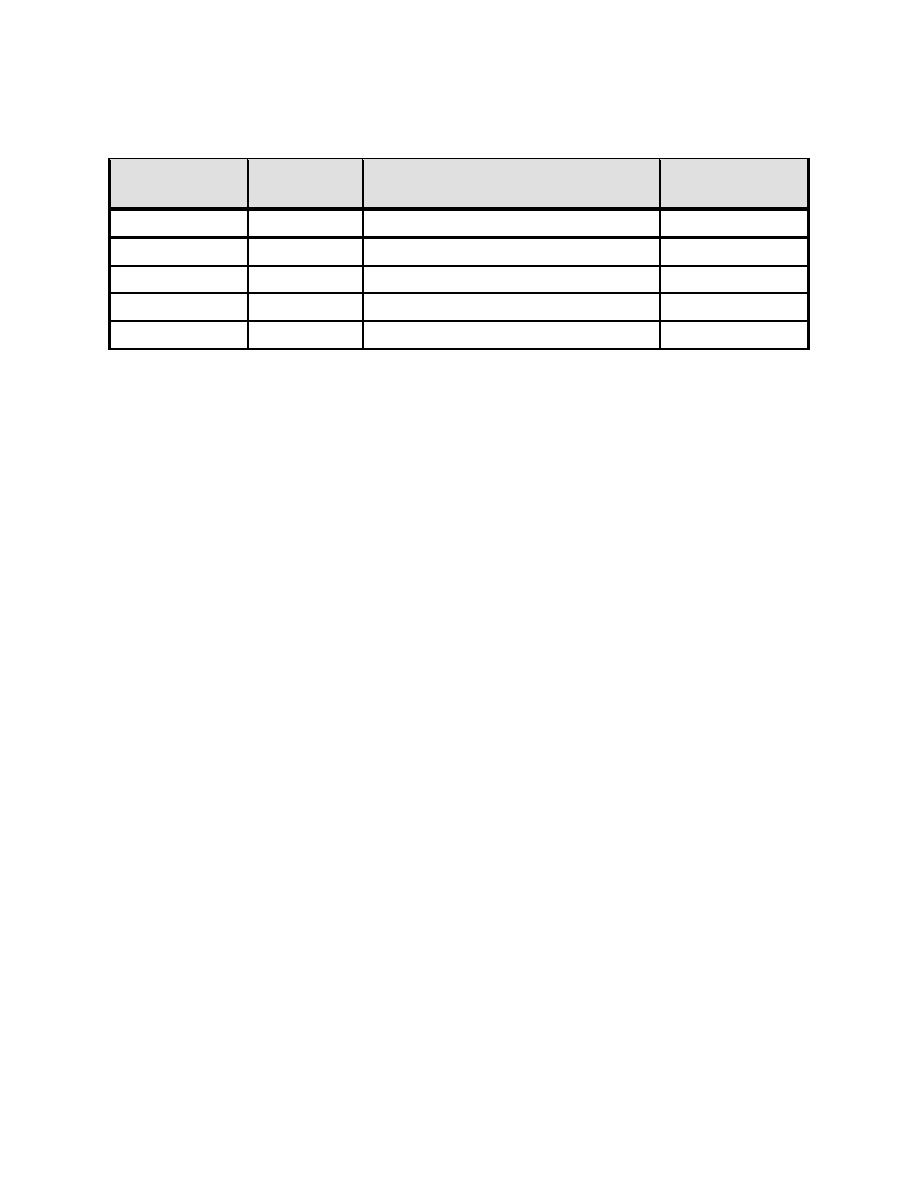
Table 6-4. Alkalinity Relationship Based on P and M Tests
Level of Alkalinity Contributed
Situation
Hydroxyl
Bicarbonate
by Carbonate
P=M
M
0
0
2P M
2 (M - P)
0
P > M
P = M
0
M
0
P < M
0
2P
M - 2P
P=0
0
0
M
EXAMPLE 6-1:
If P = 86 ppm as CaCO3, and if M = 118 ppm as CaCO3
Then, situation 2 (from Table 6-4) exists (P > M)
or P is greater than of M);
Hydroxyl = 2P - M = (2 x 86) - 118 = 54 ppm as CaCO3
Causticity = hydroxyl alkalinity as CaCO3 3
= 54 3 = 18 ppm as OH-
Carbonate = 2(M - P) = 2 X (118 - 86)
= 64 ppm as CaCO3
Bicarbonate = 0 ppm as CaCO3
Check: Total = 54 + 64 + 0 =118 ppm M alkalinity as CaCO3
Review of each situation in Table 6-4 provides this information, with situation:
1. The tests for P alkalinity and M alkalinity are equal. This means that all of
the alkalinity is due to hydroxyl ions. There is no carbonate or bicarbonate
present. (This is rare but occurs when a caustic solution is not exposed to
air.)
2. The P alkalinity is greater than one-half of the M alkalinity. This indicates
that there is hydroxyl and carbonate alkalinity, but no bicarbonate
alkalinity.
3. The P alkalinity is equal to one-half of the M alkalinity. This indicates that
all the alkalinity is due to carbonate. There is no bicarbonate alkalinity, and
the hydroxyl alkalinity is insignificant.
4. The P alkalinity is less than one-half of the M alkalinity. This indicates that
carbonates and bicarbonates are present.
166



 Previous Page
Previous Page
