UFC 3-240-13FN
25 May 2005
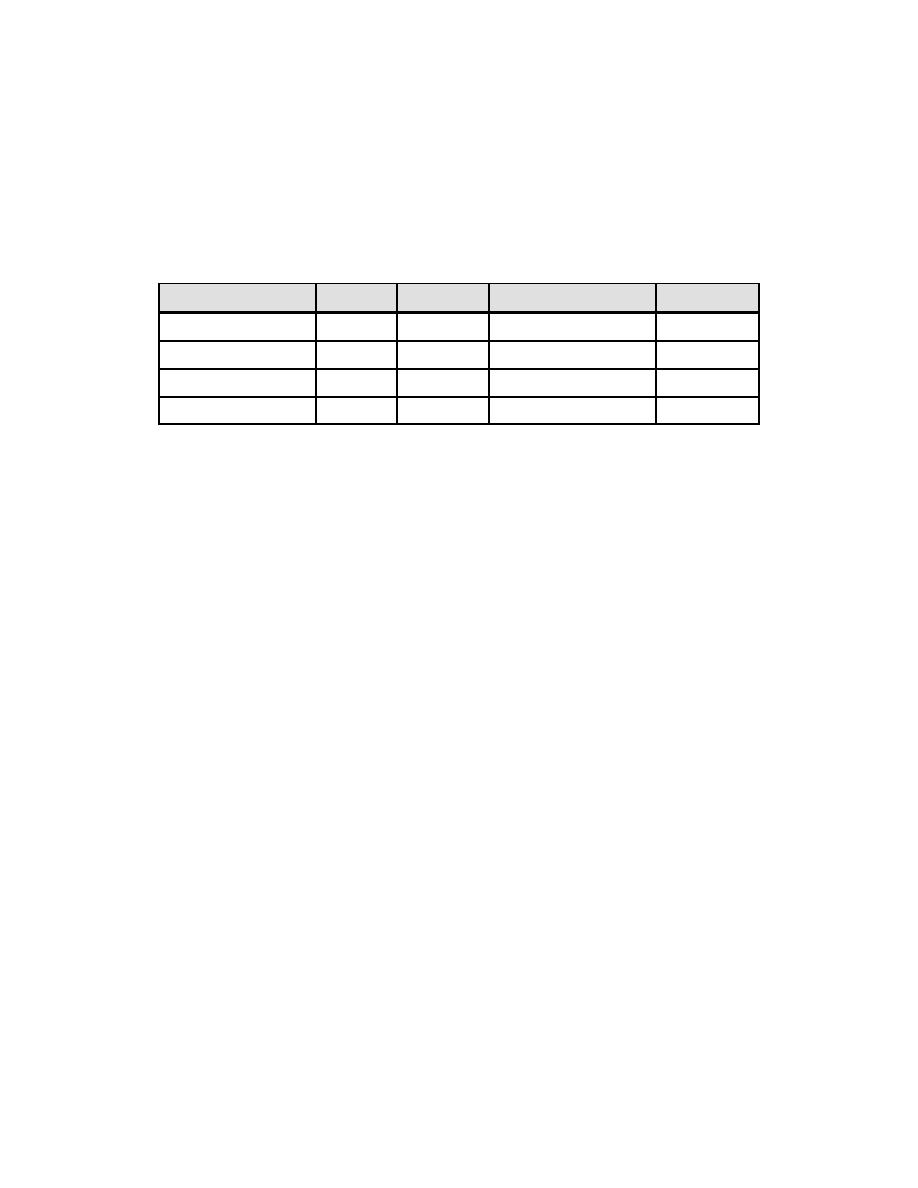
essentially unbuffered. The chemical treatment programs include coordinated
phosphate, congruent phosphate, equilibrium phosphate, and all volatile treatment.
Phosphate programs of this type are not used as conventional precipitating agents for
hardness-causing materials but instead are used as buffering agents for pH control (see
Table 3-1).
Table 3-1. Summary of Phosphate Treatment Programs
Program
PO4
OH
Na:PO4 Ratio
pH
Conventional
30-60
20-350
N/A
11-12
Coordinated
5-25
trace
2.85:1 to 3:1
9-10.5
Congruent
2-5
zero
2.3 to 2.6:1
8.8-9.4
Equilibrium
<2.4
<1.0
N/A
9.3-9.6
3-2.5.2
Pitting Corrosion. Pitting corrosion is a term that refers to a deep,
localized corrosion usually caused by oxygen molecules on the metal surfaces in the
boiler water. This process results in the formation of corrosion pits that can extend into
the interior metal layers of metal boiler components. Corrosion pitting can be severe
enough to lead to perforations of tube surfaces (see Figure 3-4).
3-2.5.3
Other Types of Corrosion. Other types of corrosion can occur in high-
pressure boilers over 6205 kilopascals (900 pounds per square inch gauge) for which
the water treatment program includes coordinated, congruent, or equilibrium phosphate-
type chemical treatment (not to be confused with standard phosphate precipitating
programs). These other corrosion mechanisms include caustic attack, hydrogen
embrittlement, and phosphate hideout.
3-2.6
Removing Oxygen from Feedwater. A very corrosive liquid results when
oxygen is dissolved in water. Oxygenated water is particularly corrosive to mild steel,
which is almost always used to construct the main components of the boiler system.
The corrosivity rate of oxygenated water doubles with every 10 oC (18 oF) increase in
temperature. Oxygen corrosion can be recognized by the presence of pits found
typically in the top of, or at the waterline of, the steam drum. Oxygen can be removed
from feedwater by mechanical or chemical methods, or both; a combination of these
methods is used commonly.
3-2.6.1
Mechanical Oxygen Removal. Mechanical removal of oxygen from
feedwater requires a deaerating heater in which both the makeup water and condensate
return are in contact with live steam and mixed using trays, sprays, or both. This heating
process literally strips most of the oxygen and other non-condensable gases out of the
feedwater. The oxygen and other gases, along with a small amount of steam, are
vented from the deaerator to the atmosphere.
54



 Previous Page
Previous Page
