TM 5-814-3/AFM 88-11, Volume III
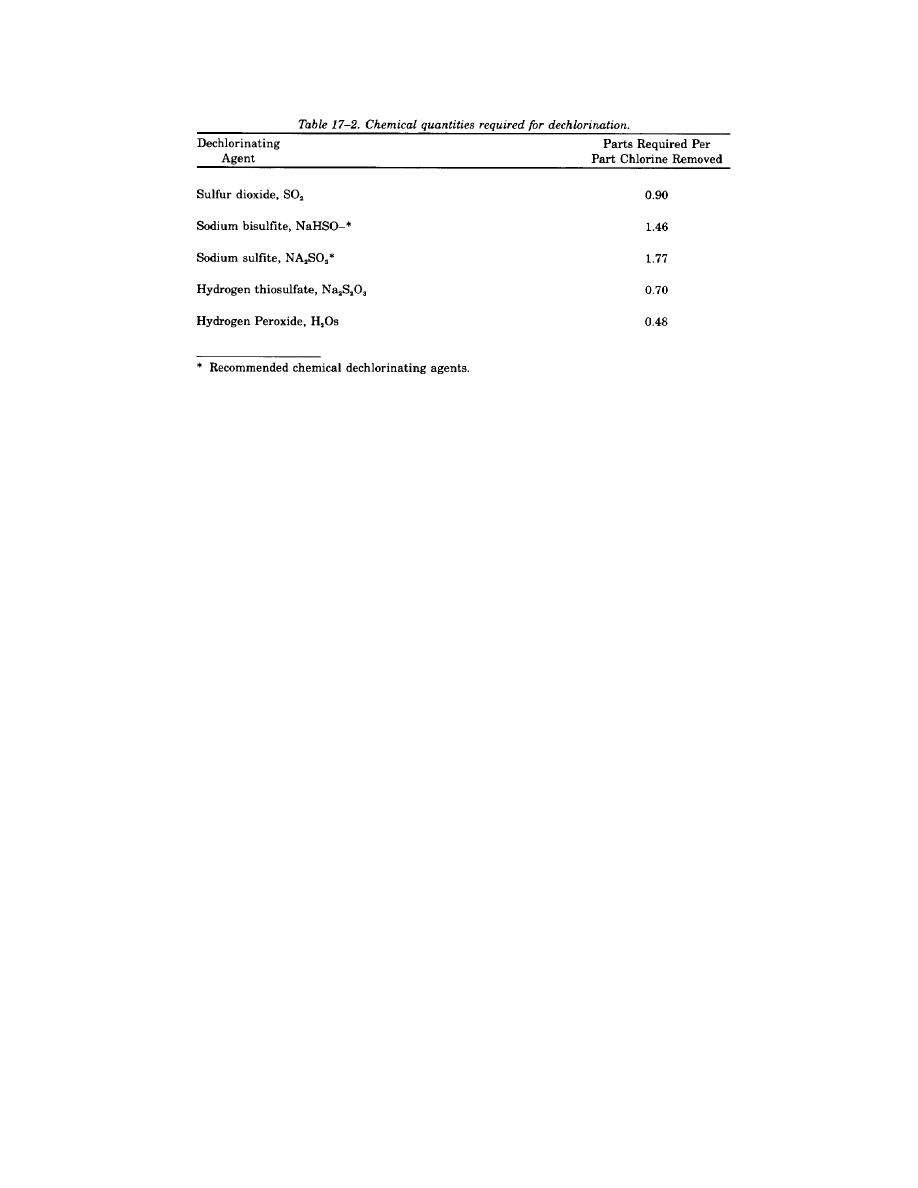
a. Sulfur dioxide. Sulfur dioxide is not flammable or explosive in either the gaseous or liquid state but,
in the presence of any moisture, it is extremely corrosive. The same materials of construction are used for
handling both sulfur dioxide and chlorine. The equipment used to meter sulfur dioxide is identical in all
respects to that for metering chlorine. Contact time is not a factor since the dechlorination reaction of sulfur
dioxide with chlorine is instantaneous, but rapid and adequate mixing at the point of application is to be
provided. The sulfur dechlorinating agents will also reduce dissolved oxygen levels, and re-aeration facilities
may have to be provided after the dechlorination step in order to satisfy permit limitation for effluent
dissolved oxygen concentrations.
b. Non-chemical methods. Non-chemical means may also be used to accomplish dechlorination. For
example, activated carbon used in granular form and a gravity or pressure-type filter bed are extremely
effective and reliable. About 0.085 part of activated carbon is needed to remove 1.0 part of chlorine.
Retention tanks or ponds may be used for dechlorination purposes but specific design criteria applicable to
all domestic wastewaters are not available because the required contact time will depend on the wastewater
plus the desired final chlorine residual. Aeration of the final wastewater effluent for the purpose of
dechlorination should be seriously considered if the pH of the wastewater is less than 8.0 and the chloramine
fraction of the total chlorine residual concentration is less than the total acceptable residual stipulated in the
discharge permit.
17-5. Ozonation.
Ozone (O3) is a particularly powerful oxidizing agent; its immediate viricidal properties are superior to those
of chlorine and to a large extent are independent of pH. As a disinfectant, it requires lower dosages and much
shorter contact times than chlorine for the same bacterial reductions. Zonated effluents have increased
dissolved oxygen concentrations and have less potential for causing a colored effluent. The high capital
requirements for ozone generation generally make such systems impractical for wastewater treatment
facilities in military installations unless dechlorination is required. Ozonation and other methods of
disinfection are covered in detail in: Gehm and Bregman, 1976; Kruse et al., 1973; Bruce et al., 1980; Fair
et al., 1966; and Parker and Bregman, 1975.
17-5



 Previous Page
Previous Page
