TM 5-852-5/AFR 88-19, Volume 5
range of values experienced in wastewater treat-
ment. Most chemical reaction rates are slower at
low temperatures. This can affect treatment and
must be considered in preparing chemical solutions
for use in wastewater treatment. The solubility of
some common treatment chemicals is given in table
9-1.
c. Flocculent sedimentation. Secondary clarifiers
and sludge thickeners generally receive relatively
high concentrations of solids and are not dependent
on temperature as predicted by Stoke's law. The
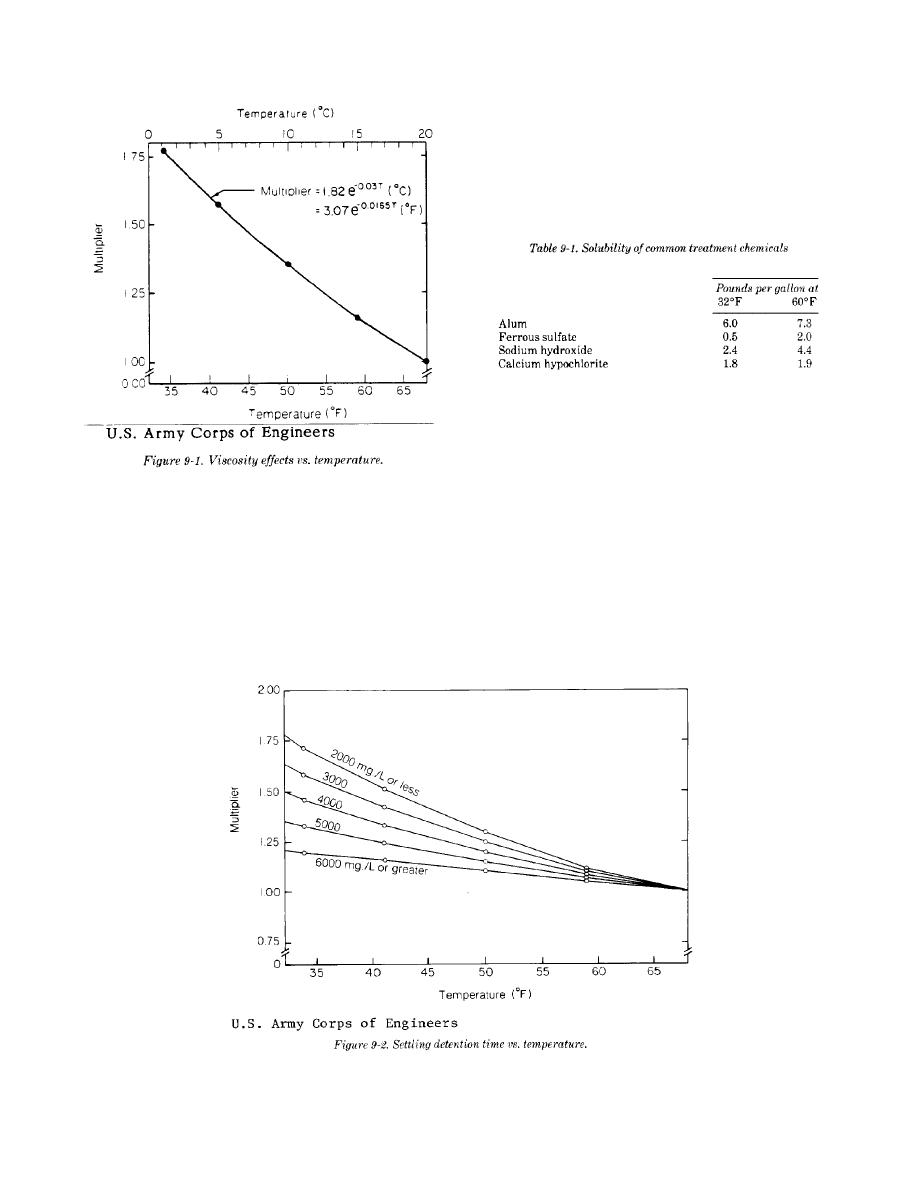
a. Gas transfer. The solubility of oxygen and
multipliers shown in figure 9-2 will be used to adjust
other gases in water increases as the liquid temper-
the size or detention time of these units, depending
ature decreases. However, the viscosity of the liquid
on the design solids concentration. At solids
also increases so that the opportunity for contact
concentrations of 2000 mg/L or less, and for
between gas bubbles and liquid molecules is
primary clarification, temperature effects are close
decreased. The net practical effect is little im-
to that predicted by figure 9-1, but as the solids
provement in overall gas transfer in cold waste-
concentration increases, the influence of tempera-
water without additional mixing.
ture decreases and figure 9-2 will be used. Density
b. Adsorption and chemical reactions. Adsorp-
currents can completely disrupt the operation of
tion is not affected by low temperatures, with the
settling tanks and thickeners, so protective ele-
9-2



 Previous Page
Previous Page
