TM 5-852-5/AFR 88-19, Volume 5
Upflow and sludge blanket clarifiers are not as
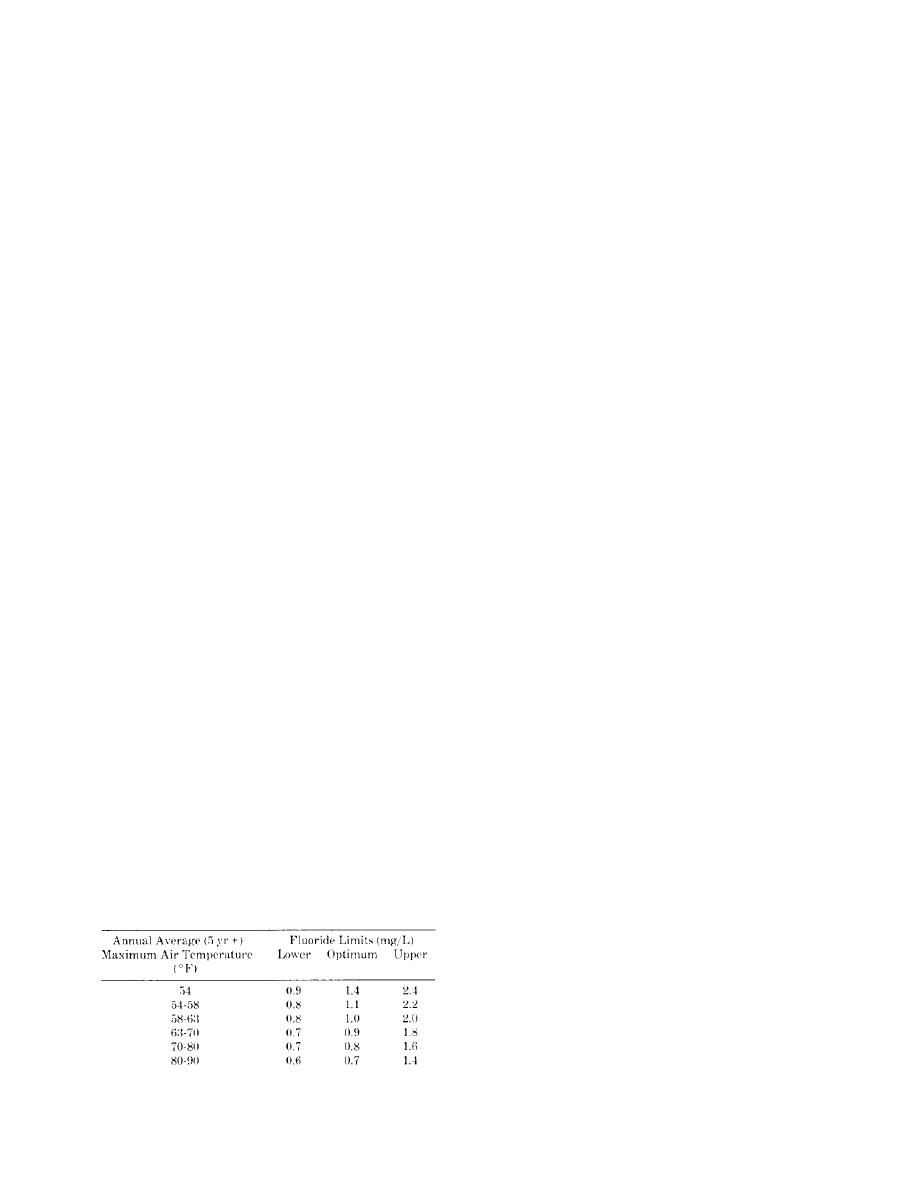
USPHS recommended fluoride limits to the annual
sensitive to viscosity effects and the multipliers from
average air temperature at the design location. (See
figure 9-2 will be used in these cases (solids
TM 5-813-3/AFM 88-10, Vol.3, for specific guid-
concentrations >2000 mg/L). Another concern for
ance at military installations.)
any type of clarifier is the presence of density
4-4. Removal of minerals and organics.
currents induced by strong temperature differences
between the incoming fluid and the tank contents.
Ion exchange water softening is commonly used at
These currents will disrupt the settling process and
smaller installations with hard water. Lime-soda
are particularly critical for upflow clarifiers. If
softening is frequently used when the water is both
possible these units will be maintained at nearly
turbid and has a high hardness. Dissolved iron is
constant temperature and the incoming fluid
common in cold regions ground waters and can foul
adjusted to that same level.
zeolite and greensand ion exchange resins so that it
c. Filtration. Filtration is influenced by low tem-
must be removed prior to ion exchange processes.
perature since the head loss through the filter is
Aeration or chemical oxidation with chlorine have
proportional to viscosity. Mixed media filters will
been successful for precipitation of elemental iron.
provide a more efficient use of space in cold regions
However, iron/organic complexes are present in
facilities. The multiplier values from figure 9-1 will
many cold regions groundwaters. Ozone has been
be used to adjust filtration efficiency. Backwashing
shown to be effective in treating such waters. Ozone
of filters is also affected since power for pumping
and carbon adsorption are very effective for color
will vary with temperature, due to the increased
and organics removal.
water viscosity. The minimum upflow velocities will
4-5. Treatment of brackish and saline
be reduced because of the increased fluid density at
waters.
low temperature.
d. Disinfection. TM 5-813-3/AFM 88-10, Vol.
Distillation, reverse osmosis and freezing have all
3, should be consulted for basic criteria on
been used in the cold regions to reduce salt concen-
disinfection procedures, chlorine dosages and
trations to potable levels.
a. Distillation. Distillation is expensive, requiring
residuals. The solubility rate of chlorine decreases at
very low water temperatures, but for practical
relatively high skill levels to accomplish, and will be
purposes this will not occur at the dosage rates
considered only if other alternatives do not exist.
b. Reverse osmosis. Reverse osmosis (RO) is
commonly used. The effectiveness of chlorination is
hindered in cold water, and the exposure times must
temperature sensitive, with best results obtained
be increased in order to provide adequate
when water temperatures are in the range of 68 to
disinfection. Contact time of about 1 hour is
85 degrees F, and the cost is also high. Packaged
recommended for cold water below 40 degrees F.
reverse osmosis units are available from about 1000
e. Fluoridation. If fluoridation is practiced at
to 1,000,000 gallon/day capacity (gpd). Power
remote cold regions facilities, the U.S. Public Health
requirements are approximately one kilowatt-hour
Service (USPHS) recommends that the dosage
of power for each 100 gallons of potable water pro-
should be increased since the actual per capita
duced. These RO systems must be protected from
consumption of drinking water tends to be some-
freezing at all times, from the point of manufacture,
what less than in temperate locations. Fluoride con-
during storage and during use.
c. Freezing. This process takes advantage of nat-
centrations of about 1.4 mg/L are recommended for
the Arctic and Subarctic. Table 4-1 relates the
ural low temperatures to separate the saline brine
from the ice which is then melted (naturally in the
spring and summer) and used for water. Trenches
have been filled with brackish water, allowed to
freeze several feet deep, and then the remaining
liquid under the ice pumped out. Spray freezing
involves sprinkling brackish water through a nozzle
to form a large cone of ice, with the brine draining
away continuously during the winter. In a pilot-scale
test in Saskatchewan, chloride content was reduced
from 2000 mg/L to 500 mg/L in the melted ice. The
recovered water represented about 75 percent of the
volume sprayed.
4-2



 Previous Page
Previous Page
