UFC 3-240-13FN
25 May 2005
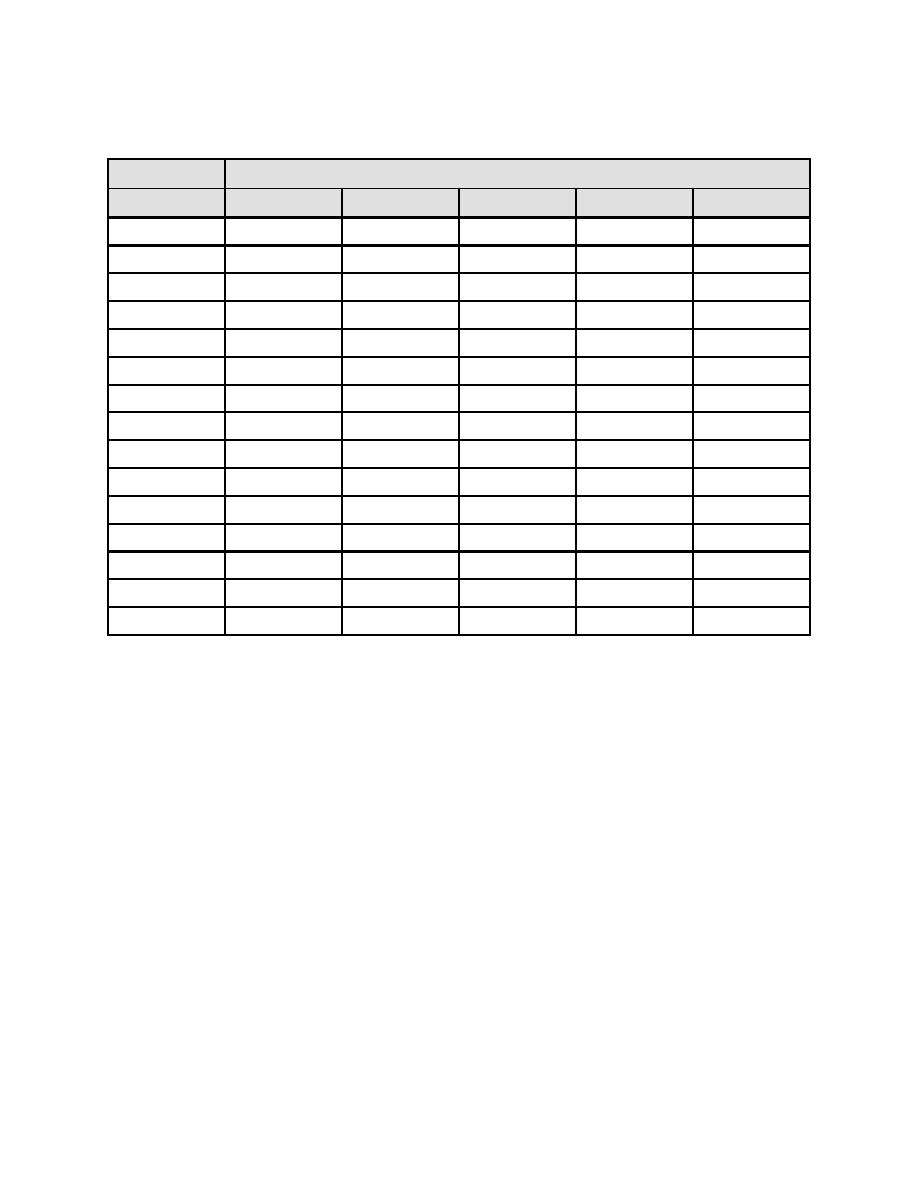
Table B-2. Factor "B" for Temperature*
F
F, Units
Tens
0
2
4
6
8
30
--
2.60
2.57
2.54
2.51
40
2.48
2.45
2.43
2.40
2.37
50
2.34
2.31
2.28
2.25
2.22
60
2.20
2.17
2.14
2.11
2.09
70
2.06
2.04
2.03
2.00
1.97
80
1.95
1.92
1.90
1.88
1.86
90
1.84
1.82
1.80
1.78
1.76
100
1.74
1.72
1.71
1.69
1.67
110
1.65
1.64
1.62
1.60
1.58
120
1.57
1.55
1.53
1.51
1.50
130
1.48
1.46
1.44
1.43
1.41
140
1.40
1.38
1.37
1.35
1.34
150
1.32
1.31
1.29
1.28
1.27
160
1.26
1.24
1.23
1.22
1.21
170
1.19
1.18
1.17
1.16
--
Find value of "B" in appropriate units column. Example: For water at 86 oF, B = 1.88
b. pHs = 9.30 + A + B - (C + D)
c. EXAMPLE B-1:
Water from a cooling tower has a TDS of 1000 ppm, calcium hardness of
500 ppm (as CaCO3), total alkalinity of 100 ppm (as CaCO3) and
measured pH of 8.2. The hottest temperature on the waterside of the heat
exchanger is 120 oF.
pHs = 9.30 + A + B - (C + D)
pHs = 9.30 + 0.20 + 1.57 - (2.30 + 2.00) = 6.77
B-2.2
Calculating pHeq
a. Puckorius and Brooke developed the improved relationship between total
alkalinity and pH after studying hundreds of cooling systems over some 20
years. The pHeq values shown in Table B-7 for the total alkalinity
measured in cooling water are used for calculating the PSI.
212



 Previous Page
Previous Page
