TM 5-813-8
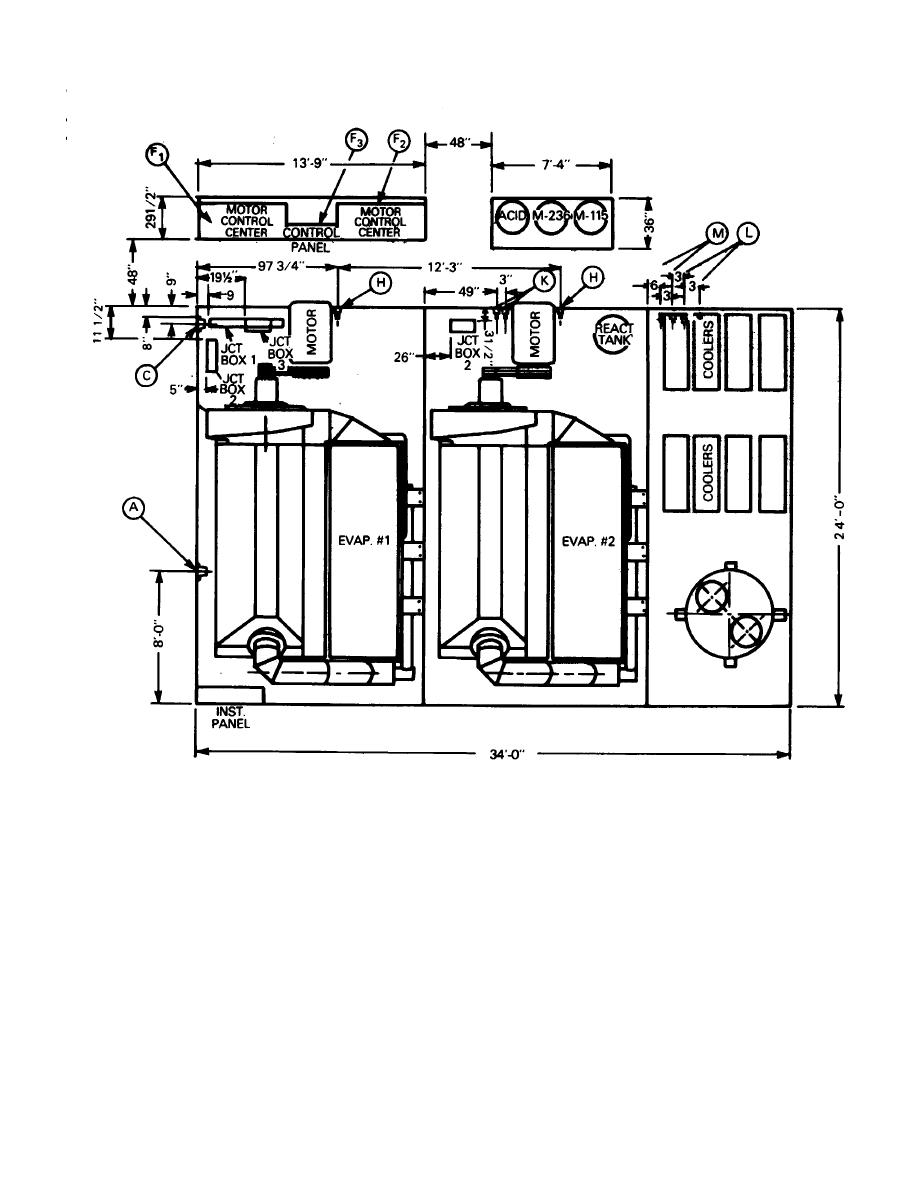
Figure A-12. Plan view of a vapor compression system.
Symbols:
++
[Ca ]
=
Double-ionized calcium concentration in moles/liter (molar)
=
=
Double-ionized sulfate concentration in moles/liter (molar)
[SO 4
-
[HSO 4]
=
Undissociated bisulfate ion concentration in moles/liter (molar)
+
[H ]
=
The dissociated hydrogen ion concentration in moles/liter (molar)
=
The negative base 10 logarithm of the solubility product
pKsp
=
The product of the concentration of the ions in a saturated solution that is beginning to form crystals
Ksp
pH
=
The negative base 10 logarithm of the hydrogen ion concentration
pOH
=
The negative base 10 logarithm of the hydroxyl ion concentration
=
The negative base 10 logarithm of the acid dissociation constant
pKa
Ka =
The ratio of the concentration of dissociated acid and hydrogen ion concentration to the undissociated
acid concentration at equilibrium
A-16



 Previous Page
Previous Page
